
Go to:

TOC
Prev
|
Plasma Cell Neoplasia
P

Malignant Plasma Cells
|
LASMA
cells are the basis for our humoral immunity--the arm of the immune system that uses circulating or secreted antibodies to neutralize abnormal or intrusive substances. As fully mature B-lymphocytes, plasma cells have acquired the complex skill of producing immunoglobulin molecules, better known as antibodies.
The name of this section thus has an oxymoronic cast: how can terminally differentiated plasma cells be malignant? Have you ever heard of cancers composed of mature nerve cells or skeletal muscle? The clue to this mystery is that malignant plasma cells have probably matured from a malignant, less terminally differentiated precursor that is their proliferating and circulating source. There are several types of neoplastic plasma cell proliferations:
Multiple myeloma (MM)
MM (images) is the best known and most common form of plasma cell malignancy, accounting for about 10% of lymphohematopoietic malignancies.
 In the U.S.A. heartland its incidence is 4 per 100,000 per year. Only 3% of the patients are less than 40 years old, and the occurence of the disease in anyone less than 30 years old must be very carefully evaluated. Radiation may play a role in some instances, since the incidence is increased in atom bomb survivors and workers at some studied nuclear reactors.
In the U.S.A. heartland its incidence is 4 per 100,000 per year. Only 3% of the patients are less than 40 years old, and the occurence of the disease in anyone less than 30 years old must be very carefully evaluated. Radiation may play a role in some instances, since the incidence is increased in atom bomb survivors and workers at some studied nuclear reactors.
As the name implies, malignant plasma cells collect in multiple foci in the bone marrow, producing lytic bone lesions detectable in skeletal surveys. These plasma cells may be morphologically identical to their benign, mature counterparts or they may have various immature or blastic features such as large nuclei, prominent nucleoli or fine chromatin (see marrow aspirate smears). In immunophenotype, like benign plasma cells the malignant ones are:
1) usually non-reactive for antigens such as CD19 and CD20 present on mature B-cells and pan-leukocyte marker LCA
2) reactive for CD38, plama cell antigen-1, and usually EMA
3) are reactive for CD56, a neural adhesion molecule, unlike benign plasma cells.
Although most MMs are aneuploid by flow cytometry, their detailed cytogenetics has been hindered by their slow growth rate.
The plasma cells secrete various products:
-
M-component:
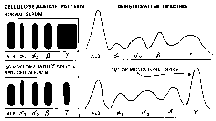 Serum Protein Electrophoresis
Serum Protein Electrophoresis |
monoclonal serum immunoglobulin. Because the plasma cells are clonal, each one secretes the same, identical immunoglobulin, called an M-component when detectable amounts accumulate in the serum. In some cases the kidneys are so ravaged that the intact immunoglobulin is no longer retained by the glomerulus, and an M-protein spills into the urine. Each M-protein has exclusively either kappa or lambda light chains and is most frequently IgG, less often IgA, and rarely another isotype. Only rare MMs fail to secrete detectable amounts of immunoprotein. The clonality or homogeneity of the M-component allows it to be detected by special studies such as serum protein electrophoresis, immunoelectrophoresis, and immunofixation (images). The latter 2 modalities are more sensitive, difficult and expensive and may be necessary to detect a small M-component.
- Bence-Jones protein
: Some MMs secrete only light chains. Unlike intact immunoglobulin, these are freely filtered by the kidney and not resorbed. Thus clonal light chains do not usually accumulate in the serum but may be detected in the urine. Some MMs secrete light chains in excess of heavy chains, so that both a serum M-component and urinary light chains are present. Bence-Jones protein, which occurs in 80% of patients, are not usually detected by routine dipstick protein assays. After a 24-hour urine sample has been concentrated, clonal Bence-Jones protein can be demonstrated by immunoelectrophoresis and immunofixation.
-
Amyloid: an altered form of the M-component seen in some MMs. It deposits in various locations, stiffening, displacing, and harming the normal tissue. It may be present in other, non-neoplastic diseases too.
-
Osteoclast Activating Factor: a functional name for several cytokines, mainly IL-1 and tumor necrosis factor. These cause much of the bone resorption and the resultant hypercalcemia.
Chief among the many baneful effects of MM are:
- Infections due mainly to decreased antibodies (the most frequent cause of death)
- Kidney disease (the second most common cause of death). Its etiology in myeloma is manifold:
- So-called "myeloma kidney", caused by waxy casts of precipitated, nephrotoxic, monoclonal light chains blocking the tubules. Lambda light-chains are more perilous than kappa ones.
- Hypercalcemia (initially present in 25% of patients) or hyperuricemia
- Amyloidosis affecting the kidney
- Renal infections
- Deposits of light chains as glomerular nodules, more often seen with kappa light chains.
- Anemia with weakness and fatigue (very common presenting findings along with pallor)
- Hypercalcemia
- Bone disease, with pain aggravated by movement and less at rest, sometimes with an associated loss of height from vertebral collapse
- Abnormal bleeding
- Serum hyperviscosity
- Amyloidosis.
With multivariate analysis, the independent prognostic factors are beta-2 microglobulin levels and plasma cell labeling index. MM is, however, an incurable disease. Despite therapy, patients die within 3 years. Indications for therapy include an increasing M protein, anemia, hypercalcemia, renal problems, lytic bone lesions, or extramedullary disease. Patients may enter a protocol study or may be offered standard treatment of either melphalan plus prednisone or a combination of alkylating agents. Few patients are eligible for allogeneic bone marrow transplantation, and even these face an early mortality of up to 25%, graft versus host disease, and myeloma relapse. Another choice is autologous transplantation.
Solitary plasmacytomas
These are the unifocal counterparts of MM. Because the volume of plasma cells is small, M-components are unusual. Some plasmacytomas occur in the marrow, where they commonly progress after years to MM. Others are extra-medullary. Especially in the upper respiratory tract, extra-medullary plasmacytomas have an excellent prognosis: after excision and local radiotherapy, most of them are cured.
Monoclonal gammopathy of uncertain significance (MGUS)
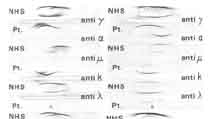
Immunoelectrophoresis (IEP)
|
MGUS is identified by a small serum M-component produced by a minor clone of plasma cells that may not be detectable by bone marrow biopsy. The most important characteristic is a serum M-component < 3 g/dl that doesn't increase with time. Additional features include:
• < 5% plasma cells in the marrow
• absence of lytic bone lesions, renal insufficiency,
anemia, or hypercalcemia
• urine with little or no M-protein
MGUS is a phenomenon of the elderly and is increasingly frequent with age, seen in about 3% of people older than 70 years. The qualifier "uncertain significance" betrays our ignorance about the condition's malignant potential in individual patients. In one large study 241 patients with MGUS were followed for a median of 22 years:
|
Status After Average 22 Years |
Percent |
| No progression of M-component | 19% |
| Developed malignant immunoproliferative disease; MM in 16% |
24% |
| Died of unrelated causes |
47% |
| M-component became > 3 g/dl but no chemotherapy needed |
10% |
Depending on the level of the MGUS M-component, guidelines exist for appropriate follow-up. If the M-component is < 2 g/dl, it is measured by an SPEP again in 6 mos and, if stable, annually thereafter. Bone marrow exam and imaging studies are called for only in the event of additional abnormalities.
Sometimes it is hard to say whether a patient has a MGUS on the one hand or, on the other,
MM or Waldenstrom's macroglobulinemia. With some exceptions the following features are suggestive of malignancy:
- M-protein > 3 g/dl
- Plasma cells > 10% of marrow cellularity
- An elevated plasma cell labeling index
(a measure of the cells' proliferative tendency)
- Monoclonal light chains accompanying the M-protein.

Table of Contents
|
Previous section
|


