
Go to:

TOC
Prev
Next
|
Immunophenotyping
Lymphomas
IMMUNOPHENOTYPING refers to the technique
of identifying molecules that are associated with lymphoma cells and that help to characterize them. The molecules are identifiable
because, in almost all analyzable cases, they are expressed on the outer
cell surface membrane. The molecules are identified by using special antibodies that
bind to them specifically . In this context these molecules
are called "antigens," and the specific part of the molecule to which the
antibody attaches is called the "epitope".
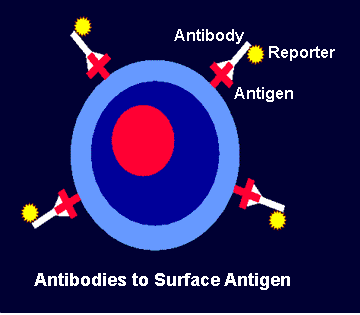
The antibodies themselves have
been manipulated so that they bear artificial reporter moieties.
The technique is called "immunophenotyping" for 2 reasons: 1) It is
dependent on the activity of antibodies, which are immunological substances
2) It is used chiefly to identify lymphoid and hematopoietic cells, which
are part of the immune system.
How It Works:
-
A solution of
antibodies (called serum) is poured over the lymphoma cells, which may
be in liquid suspension or may be in a very thin section of tissue on a
slide.
-
If the lymphoma
cells bear the molecules (antigens) for which the antibodies are specific,
the antibodies bind to the cells.
-
The cells are
washed to prevent non-specific binding; the specific antibodies remain
bound.
-
The cells with the bound antibodies are manipulated so that the reporter
moiety attached to the antibodies declares its presence. This can be done
several ways:
-
Immunofluorescence: The reporter
molecule may fluoresce when exposed to light of the appropriate frequency.
If the specimen is a tissue section, it can be examined under a fluoresent
microscope, and cells with bound antibodies will glow in the dark (like
Halloween).
-
Flow cytometry:
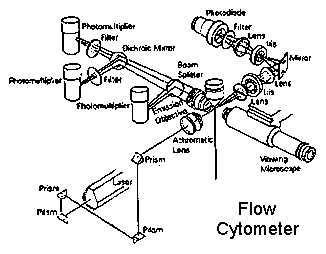
If the specimen
is cells suspended in liquid, the cells can be run through a flow cytometer.
This will expose cells in a stream of liquid one at a time to a beam of
laser light. If they bear the right bound antibodies, they will fluoresce
and the cytometer will record the signal.
-
Immunohistochemistry: Sometimes
cells in a tissue section are identified with antibodies that have attached
enzymes (usually horse radish peroxidase or alkaline phosphatase). When
the tissue section is exposed to an appropriate chromogenic substrate,
the enzyme on the bound antibodies will cause the substrate to change color
and precipitate on the cells.
How the Antibodies Are Made:
-
Animals (mice, rabbits, goats) are immunized
with the antigen for which a corresponding antibody is desired. Serum from
the immunized animals is collected and purified. The antibodies thus procured
are called "polyclonal antibodies". Because they are naturally made,
they consist of many different kinds of antibodies with varying degrees
of specificity for the antigen.
-
Alternatively, through the miracles
of modern science (invent anything this insanely great, and your mailbox will contain a Nobel Prize),
individual cells that secrete the desired antibody can be isolated from
immunized animals. The selected cell is then fused with a neoplastic plasma
cell that also has a natural propensity to make antibodies. This fused
hybrid, called a "hybridoma" cell, secretes only one kind of antibody,
without the variation seen in the first method above. The serum from the
hybridoma cells thus is rich in a "monoclonal antibody".
What Good Is Immunophenotyping: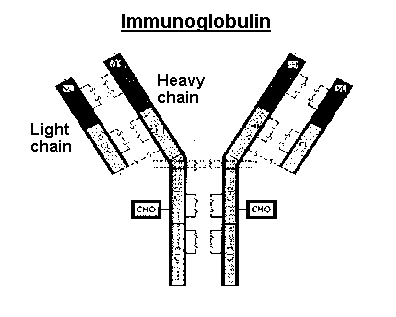
Immunophenotyping is used clinically chiefly to diagnose and categorize lymphomas and leukemias.
Immunophenotyping is helpful because in many instances different kinds of benign and malignant
lymphoid cells resemble each other in routinely stained tissue sections
and smears. For example the small cells of small lymphocytic lymphoma are dead-ringers for benign small lymphocytes. (Can you tell them apart?) When lymphoma is suspected, the technique helps to distinguish
between malignant and benign lymphoid proliferations, between B- and T-cell processes,
and between sub-categories of B- and T-cell lymphomas (see this technique illustrated in a normal node).
-
It is especially useful in the case
of B-cell lymphomas that express surface immunoglobulin (and most of them
do). Immunoglobulin molecules contain a light chain and a heavy chain.
In a random, benign collection of lymphoid cells, the kappa light chains are present on roughly 2/3rds of the cells and lambda light chains on 1/3rd. If you applied an antibody to kappa light chains,
it would mark 2/3rds of the cells; an antibody to lambda would mark 1/3rd.
On the other hand, a malignant collection of lymphoid cells is monoclonal,
at least at first approximation. By definition, then, all the cells will
bear identical surface immunoglobulin molecules with either kappa or lambda light chains, but not a mixture. Thus an antibody to a specific kind of light chain (kappa or lambda) will mark
either all or none of the cells.
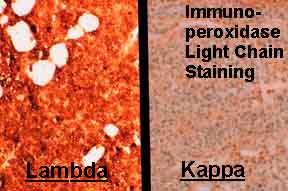
The image above shows immunoperoxidase light chain staining, where each of 2 frames represents the same tissue stained with antibodies to different light chains. All the cells stained by lambda antibody are positive (deep orange).
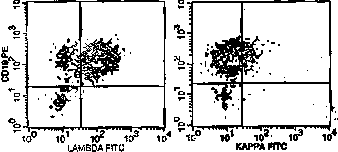
Another illustration shows the equivalent study done by flow cytometry. The cloud of positive events in the lambda frame has been shifted significantly left (positively) compared with the kappa frame.
-
Antibodies can also distinguish between B- and T-cells because these cells bear distinct
kinds of surface molecules.
-
Furthermore, because specific subtypes
of lymphomas are at least partly distinguished by their
surface molecules, antibodies to these molecules can help tell them apart.
Cluster Designation Numbers:
Researchers in many scattered laboratories painstakingly identified many lymphoid surface
antigens and developed antibodies to them. Every
so often, in world congresses, it would become apparent that antibodies
originating from different labs and bearing different names were
marking the same antigen/molecule. At that point, the antigen would be assigned
a cluster designation, or CD number, meaning that a known cluster of antibodies were binding to this known antigen. Any of these antibodies might be referred to by one of several idiosyncratic laboratory names or by the CD number of the antigen. For example, antibodies that recognize CD20, a characteristic B-cell molecule, might be called alternatively L26, B1, or, by association, CD20. This is very confusing and is one reason why hematopathologists keep lots of aspirin in their desks. Nonetheless, if you study lymphomas, you will have to know at least the basics of this scheme. Here are the basics:
-
All lymphoid cells (well, almost
all): Lymphoid cells are reactive for CD45
(leukocyte common antigen, or LCA).
-
B-cells: Almost all of these
are reactive for CD19, CD20
and CD22 (remember them as the college-age
markers). Certain low-grade B-cell lymphomas are reactive for two markers
otherwise found only on T-cells: CD5 and CD43.
Follicular center cell lymphomas (as well as very different fish, lymphoblastic
lymphomas) are frequently CD10(+).
-
T-cells: Pan T-cell markers (present
on almost all T-cells) include CD2, CD3,
CD5, and CD7
(the early childhood markers). Most T-cells mark with either CD4
(helper cells) or CD8 (suppressor cells or
cytotoxic cells).
-
Natural-killer cells: These tough
guys are frequently associated with CD16,
CD56, or CD57.
There are spectacular tables of CD antibodies at the University of Washington, or for those with a taste for things British at Birbeck College in the UK.

Table of Contents |
Next section |
Previous section
|


