
Go to:

TOC
Prev
Next
|
Classifying Non-Hodgkin's Lymphomas
CLASSIFYING non-Hodgkin's lymphomas makes sense for several reasons:
- The categories appear to correspond to biological
entities that behave distinctly. Thus the pathologist gives
the clinician important guidance for treating the lymphoma and assessing its
prognosis.
- A knowledge of the features of each category helps the pathologist to
recognize a lymphoma. Since
lymphomas can assume a bewildering variety of appearances, it's helpful to
know what the coherent patterns are.
- By observing how the lymphomas group themselves, one can discover
important biological principles that underlie their appearance and behavior.
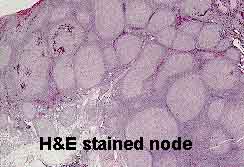
Pathologists have traditionally depended heavily on the morphologic appearances of lymphomas
to categorize them. Twenty years ago, morphology was the only tool available.
Suspicious lymphoid tissue was (and still is) fixed in formalin or a mercury-containing fixative, embedded in paraffin, sliced very thinly (5 microns or less), placed on a glass slide, and stained with the all-purpose tissue stain, hematoxylin and eosin. The earliest attempts to categorize lymphomas relied solely on this method.
Starting in the 1970's other techniques have been developed to study the nature of both benign and malignant lymphoid cells.
- Immunophenotyping
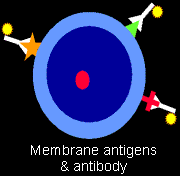 : Different types of lymphoid cells express different
molecules on their surface cell membrane. Clever scientists enhance their careers by
making antibodies that will adhere specifically to these molecules,
in this context called antigens. If the
antibodies are altered in special ways so their presence can be detected
(for example, they may be rendered fluorescent), this technique can be used
to assess what kinds of antigens decorate the cell membrane. These antibodies are eventually given so-called "cluster designation" or "CD" numbers.
Immunophenotyping has become important in evaluating 1) the malignancy of a
lymphoid proliferation and 2) the lymphoma category to which it belongs.
Three methods of immunophenotyping that yield the similar information are: : Different types of lymphoid cells express different
molecules on their surface cell membrane. Clever scientists enhance their careers by
making antibodies that will adhere specifically to these molecules,
in this context called antigens. If the
antibodies are altered in special ways so their presence can be detected
(for example, they may be rendered fluorescent), this technique can be used
to assess what kinds of antigens decorate the cell membrane. These antibodies are eventually given so-called "cluster designation" or "CD" numbers.
Immunophenotyping has become important in evaluating 1) the malignancy of a
lymphoid proliferation and 2) the lymphoma category to which it belongs.
Three methods of immunophenotyping that yield the similar information are:
1) immunohistochemistry
2) immunofluorescence
3) flow cytometry.
- Cytogenetics: Like all cells, malignant lymphoid cells can be made to
proliferate in vitro, and their metaphase chromosomes can be examined for
characteristic translocations. It is encouraging to the morphologically oriented
hematopathologist that his or her careful microscopic observations very frequently
correspond to genetic distinctions uncovered by "scientific" techniques. As Oscar Wilde said, only very superficial people are uninterested in surface appearances.
- Molecular analysis:
This technique is usually geared toward finding clonal
(neoplastic) rearrangements of the immunoglobulin gene in B-cell malignancies or of
the T-cell receptor gene in T-cell malignancies. These rearrangements are too subtle to be detected by conventional cytogenetics.
Most classifications are based on the assumption that lymphoma cells are the
malignant counterparts of benign lymph node cells.
The various lymphomas are often named after the
benign cell from which they are assumed to derive.
Rappaport Classification
The
oldest classification that still crops up is the Rappaport
classification, which was developed before lymphoid cells
were divided into B-cells and T-cells. Occasionally the following terms may be heard:
- Well-differentiated lymphocytic lymphoma = small lymphocytic lymphoma.
- Poorly differentiated lymphocytic lymphoma = follicular center
cell lymphoma with a large component of small-cleaved cells.
- Histiocytic lymphoma = large cell lymphoma
Kiel and Lukes & Collins Classification
A gala year for classifications, 1974 saw the introduction of 2 new ones. (In fact the diversity and complexity of classifications had reached the point that one British physician was moved to publish a parody in The Lancet.)
The Kiel Classification is popular in Europe.
The Lukes and Collins Classification, which was
the first to separate B-cell and T-cell lymphomas using immunologic techniques,
has been popular in the United States. Some of the terminology from both
classifications has made its way into the lingua franca of hematopathology.
Working Formulation
By the early nineteen-eighties, so many classifications and systems had
proliferated that a large study was initiated to separate the sheep from the goats
(i.e., tell which systems were valid). Investigators at the National Cancer Institute
looked at 1175 cases of non-Hodgkin's lymphoma and concluded that each of the
classifications had clinical value but none was clearly superior.
True hematopathologists, they therefore invented yet another classification, a meta-classification called
the Working Formulation. It is important (or at least
interesting) to remember that this grouping:
- was originally intended to translate among the previous classifications, not to replace them.
- was based solely on the morphology of H&E stained sections.
- groups the lymphomas into morphologic categories that may encompass
several individual diseases.
Despite these drawbacks, the Working Formulation probably provides
the most common convenient terminology for discussing lymphomas. The Formulation's
categories do have clinical validity (therapeutic and prognostic), are based on
relatively simple morphologic features, and for this reason offer diagnostic criteria
that are reproducible among pathologists.
The criteria are both architectural (low magnification) and cytological
(high magnification):
- Architectural
- diffuse proliferation
- follicular proliferation
- Cytological
- Nuclear outline
- cleaved (indented)
- non-cleaved
- Cell size
- small
- large
- mixed small and large
Note that there is no consideration of B or
T-lineage. Using data from the study of the original 1175 cases, the
Working Formulation entities are divided into low, intermediate, and
high grade lesions. This is the information that is most important to the
treating clinician.
|



